- 欢迎来到北京优尼康生物科技有限公司
- |
-
商品分类

 010-64814275
010-64814275

 010-64814275
010-64814275

INTENDED USE:
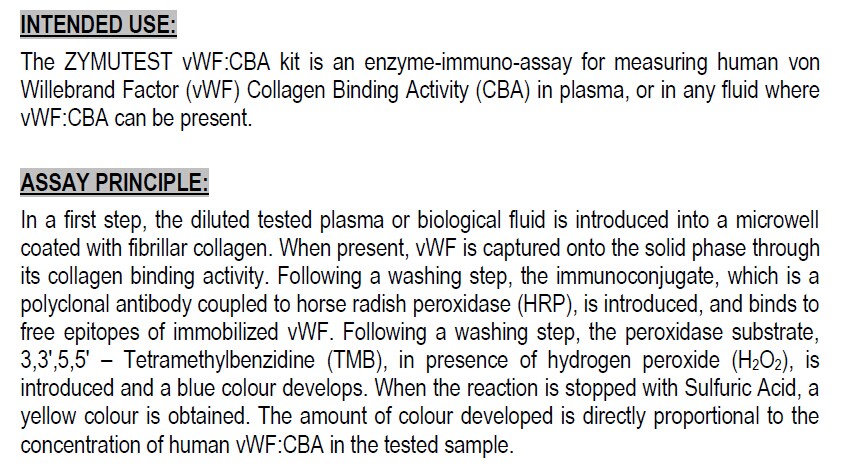
The ZYMUTEST vWF:CBA kit is an enzyme-immuno-assay for measuring human von Willebrand Factor (vWF) Collagen Binding Activity (CBA) in plasma, or in any fluid where vWF:CBA can be present. ASSAY PRINCIPLE:
In a first step, the diluted tested plasma or biological fluid is introduced into a microwell coated with fibrillar collagen. When present, vWF is captured onto the solid phase through its collagen binding activity. Following a washing step, the immunoconjugate, which is a polyclonal antibody coupled to horse radish peroxidase (HRP), is introduced, and binds to free epitopes of immobilized vWF. Following a washing step, the peroxidase substrate, 3,3',5,5' – Tetramethylbenzidine (TMB), in presence of hydrogen peroxide (H2O2), is introduced and a blue colour develops. When the reaction is stopped with Sulfuric Acid, a yellow colour is obtained. The amount of colour developed is directly proportional to the concentration of human vWF:CBA in the tested sample. TEST SAMPLE:
Trisodium Citrate anticoagulated human plasma.
Any biological fluid where vWF:CBA must be measured. REAGENTS:
1. COAT: Micro ELISA plate, containing 12 strips of 8 wells, coated with equine collagen (types I and III), then stabilised; the plate is packed in an aluminium pouch hermetically sealed in presence of a desiccant.
2. SD: 2 vials containing 50ml of Sample Diluent, ready to use.
3. Cal: 3 vials of vWF Calibrator, lyophilised. When restored with 2 ml of Sample Diluent, a plasma containing a concentration “C” (expressed in %) of human vWF:CBA is obtained. This concentration (in the range 120-160% according to the lot), established by reference to the NIBSC international standard, is accurately determined for each lot.
4. CI: 1 vial containing 0.5 ml of lyophilised vWF Plasma Control I (High) (human plasma).
5. CII: 1 vial containing 0.5 ml of lyophilised vWF Plasma Control II (Low) (human plasma).
Note: The vWF:CBA concentrations and acceptancy ranges for control plasma I and II, and calibrator, can vary from lot to lot, but are precisely indicated for each lot on the flyer provided in the kit.
6. IC: 3 vials of Anti-(h)-vWF-HRP immunoconjugate, a polyclonal rabbit antibody specific for human vWF, coupled to Horse-Radish-Peroxidase (HRP), lyophilised.
7. CD: 1 vial of 25 ml of Conjugate Diluent, ready to use.
8. WS: 1 vial of 50 ml of 20 fold concentrated Wash Solution.
9. TMB: 1 vial of 25 ml peroxidase substrate: 3,3',5,5' – Tetramethylbenzidine containing hydrogen peroxide. Ready to use.
10. SA: 1 vial of 6 ml of 0.45M Sulfuric acid (Stop solution). Ready to use.
Note: Use only components from a same lot of kits. Do not mix components from different lots of kits, when running the assay. REAGENTS AND EQUIPMENT REQUIRED BUT NOT PROVIDED:
- 8-channel or repeating pipette allowing dispensing 50-300 μl.
- 1-channel pipettes at variable volumes from 0 to 20 μl, 20 to 200 μl and 200 to 1000 μl.
- Micro ELISA plate washing equipment and shaker.
- Micro ELISA plate reader with a wavelength set up at 450 nm.
- Distilled water.
REAGENTS PREPARATION, STORAGE AND STABILITY:
In their original packaging box, before use, when stored at 2-8°C, the unopened reagents are stable until the expiration date printed on the box.